Balancing Net Ionic Redox Equations
Now click the button Balance to get the equalize equation. Remember that a redox reaction must be balanced for the number of electrons exchanged as well as for number of atoms on each side.
Introduction to acidbase reactions.
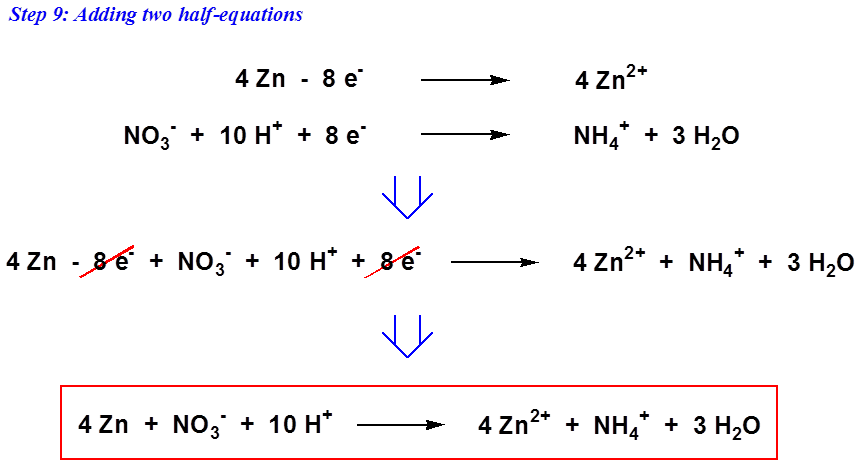
. Add the two half-reactions together and cancel out common terms. __CrO42 __C6H6 __Cr3 __CO2. Full reaction balanced in acid.
Identify all of the phases in your answer. Combining the half-reactions to make the ionic equation for the reaction. The balancing redox reactions calculator tells whether a reaction is actually a redox reaction or not.
Check that both sides of the equation have the same kind and number of atoms as well as the same charges. Balance the Redox Reaction in Acidic Medium by Half Reaction Method. Write balanced net ionic equations for the reaction that takes place between H2O2 aq and MnO-4 all numbers are subscripts of the respective element with the.
Identify which atoms are oxidized and which are reduced. Textbook Solutions Expert Tutors Earn. Finally for the specified chemical equation a window will pop with the output.
It winds up with the equation balanced in basic solution. Ive been working on this problem for about 30 minutes and Im still unable to achieve an answer. What we have so far is.
For the reaction between NO 2 and H 2 the net charge on both sides of the equation in Step 1 is zero. Use uppercase for the first character in the element and lowercase for the second character. Balancing Redox Equations 2 Balance each of the following net ionic equations for a redox reaction.
Balancing a Net Ionic Redox Equation step-by-step 1. Use the rules to assign oxidation numbers to all atoms in the equation. Because the charge and the atoms are balanced the equation is correctly balanced.
Draw a line connecting the atoms involved in oxidation and another line connecting the atoms involved in reduction. Solution for Balance the net ionic redox equations by the half-reaction method. Any help would be greatly appreciated.
Balance the following equations of redox reactions. The following unbalanced net ionic equation provides an example. Part A VO2aqHaqClO2aqVO2aqClO2gH2Ol Express your answer as a net ionic equation.
Cr 2 O 7 2 Cl --- Cr 3 Cl 2 O 2 there is a minimum of 2 Crs 2Cr 6 6e --- 2Cr 3. How to use Net Ionic Equation Calculator. This final check confirms that the equation is perfectly balanced in terms of atoms and charges.
ELECTROCHEMISTRY Electrochemistry BALANCING NET IONIC EQUATIONS FOR REDOX REACTIONS Writing ionic. Balance the atoms in each half reaction. Add 6 electrons to the left-hand side to give a net 6 on each side.
Balancing Redox Equations 1. Cr 2 O 72- 14H 6e - 2Cr 3 7H 2 O. Balancing net ionic redox equations The simplest way to express a redox reaction is an equation that shows only the oxidation and reduction processes.
Balancing Redox Equations for Reactions in Basic Conditions Using the Half-reaction Method. Identify all of the phases in. Solution for Balance the net ionic redox equation by the half-reaction method in acid and in base.
Method to use the Ionic net equation calculator is as follows. Fe Au Co Br C O N F. In order to understand how this is done consider the balanced equation for the reaction of ironII nitrate and.
6 I once saw an unusual method to balancing this particular example equation. Replace immutable groups in compounds to avoid ambiguity. Now all that needs balancing is the charges.
First we balance the molecular. View Balancing Net Equations for Redox Reactionspdf from CHEMISTRY 031 at Malayan Colleges Laguna. Similarly it also balances the number of charges ions and atoms at both sides of the equation to help you understand the reaction more easily.
There are three main steps for writing the net ionic equation for Zn CuNO32 Cu ZnNO32 Zinc Copper II nitrate. 5 A more detailed discussion about balancing this equation can be found here. ZnsAl3aqZn2aqAls Express your answer as a net ionic equation.
The net ionic equation is thus obtained. Balance each of the following net ionic equations for redox reactions. Balancing a redox equation in acidic solution.
Here it is in all its glory. For example C6H5C2H5 O2. __Fes __ClO4 aq __Fe3aq __Cl aq.
The balanced equation will be calculated along with the states complete ionic equation net ionic equation and spectator ions. Assign oxidation numbers to all elements in the reaction. Enter the chemical equation in the Enter the chemical equation field.
You just have to insert the equation and the calculator will display oxidation and reduction. Balancing a redox equation in basic solution. Separate the redox reaction into two half reactions.

Redox Reactions Part 4 Writing Net Ionic Equations Youtube
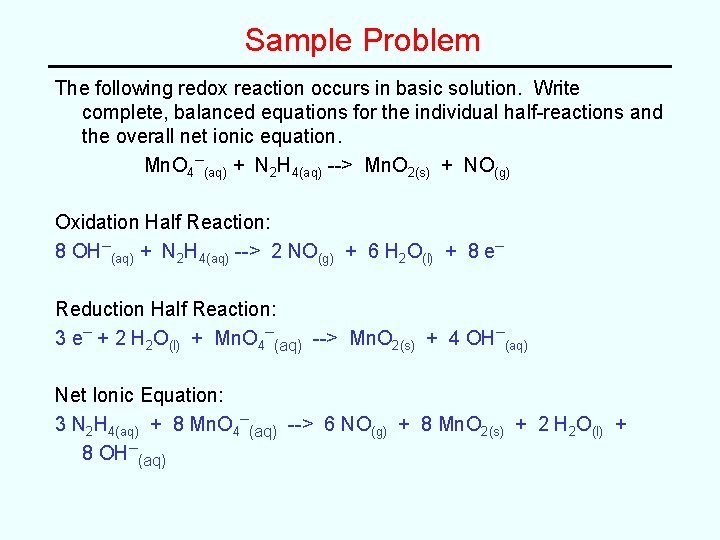
Oxidation Reduction Reactions Redox Terminologyreview Chapter 4 Redox
Oxidation Reduction Reactions Redox

9 1 Writing Net Ionic Equations Sl Youtube
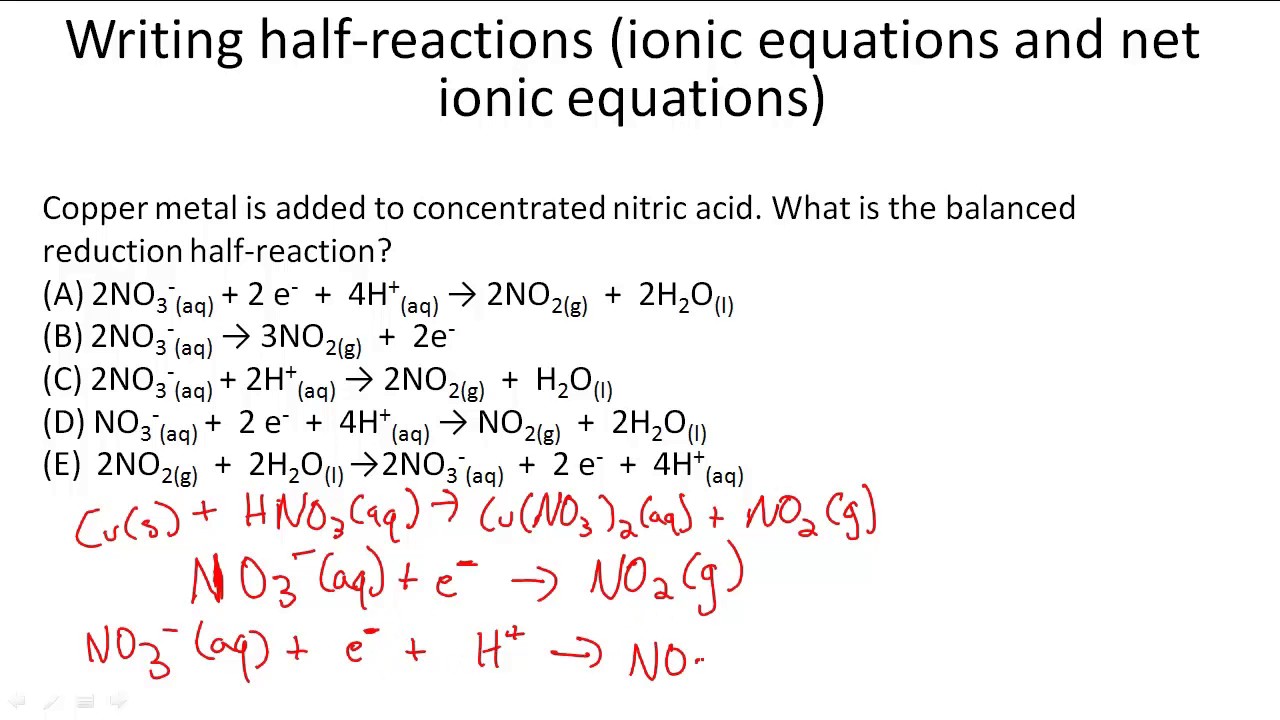
Writing Half Reactions Ionic Equations And Net Ionic Equations Youtube
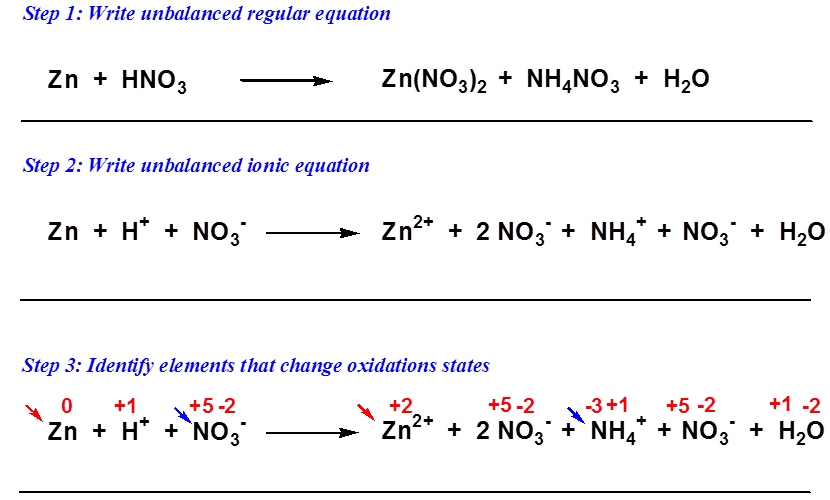
0 Response to "Balancing Net Ionic Redox Equations"
Post a Comment